INDICATION
ABECMA (idecabtagene vicleucel) is a B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
This website is best viewed using the horizontal display on your tablet device.

This website is best viewed using the vertical display on your mobile device.
Proven CAR T Cell Therapy Power in the Patients You’re
Likely to See1
Rapid, deep, and durable responses with ABECMA®1-3*
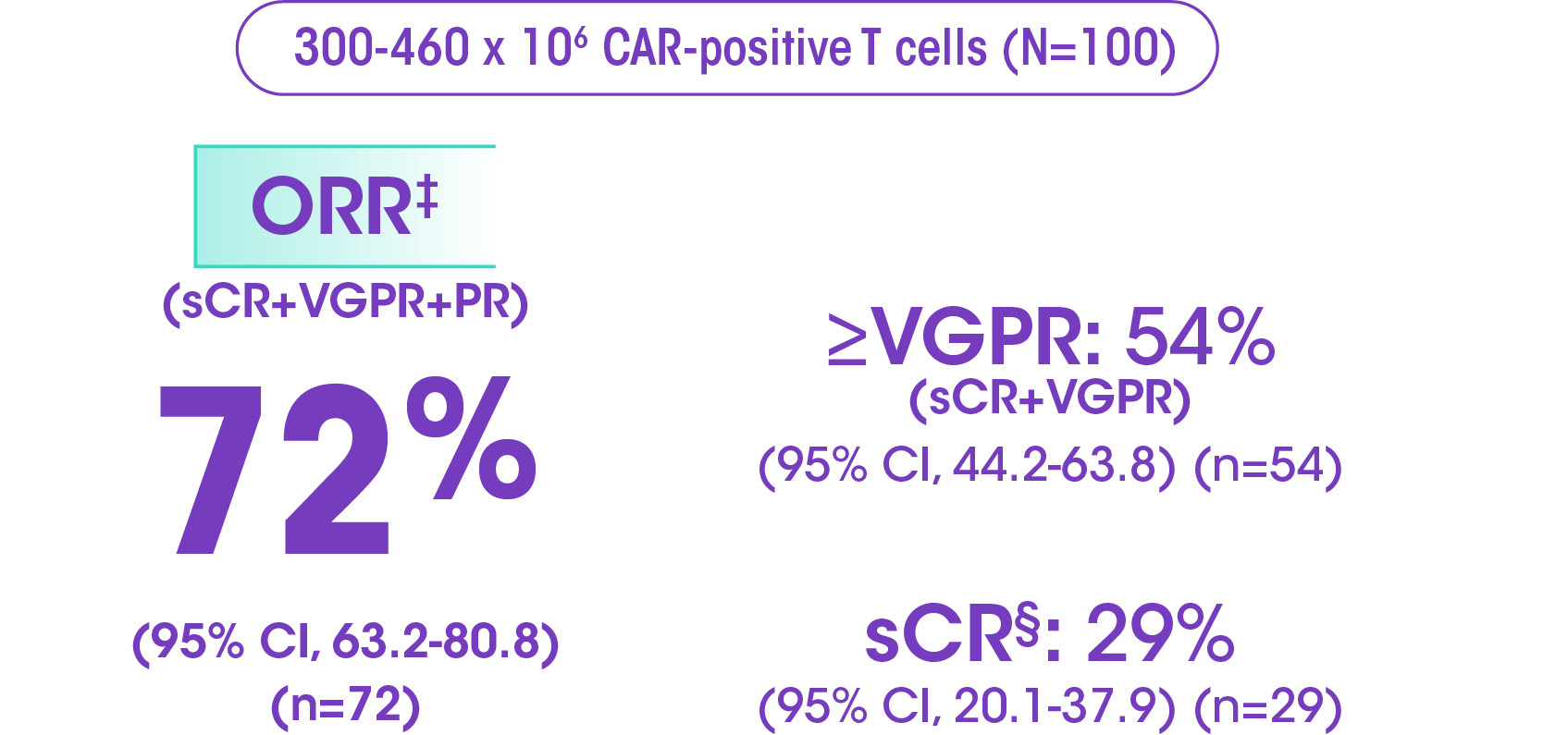
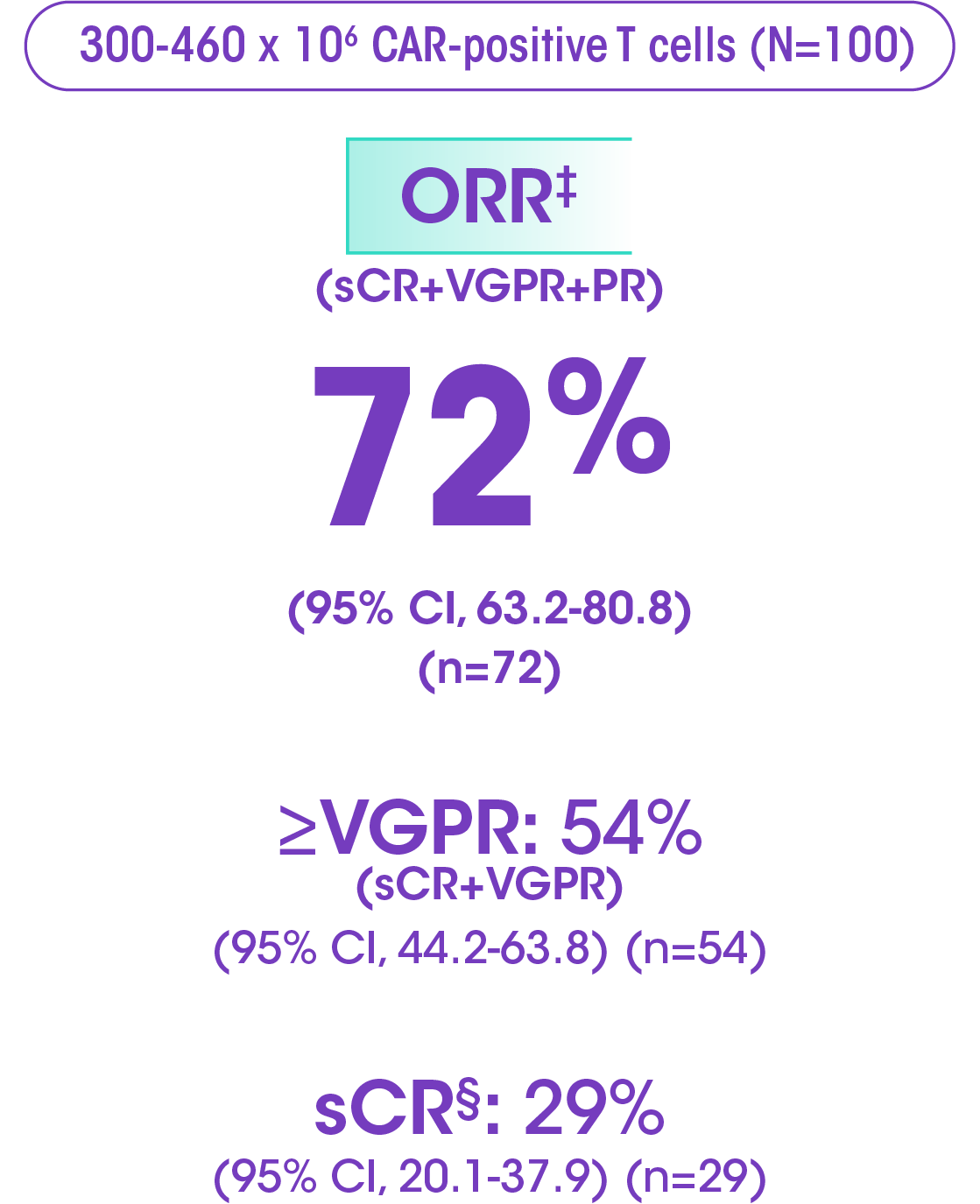
ORR at primary analysis (median follow-up of 13.2 months, range: 0.2-21.0): 72% ORR (95% CI, 62-81) (n=72)†
Deep responses were achieved in MRD-evaluable patients with ≥VGPR2
93% MRD NEGATIVITY in MRD-evaluable patients||
(n=39)
- In KarMMa, 54 patients had ≥VGPR
- Of those patients, 42 were evaluable for MRD
- 93% of evaluable ≥VGPR patients were MRD negative (39/42 patients)
A majority of patients responded to ABECMA, with more than half achieving ≥VGPR
- mTTR: 1 month (range: 0.5-2.9 months; n=72)
- mDOR‡: 11.3 months (95% CI, 10.3-15.3; n=72)
- mDOR with ≥CR: 21.6 months (95% CI, 13.5-NE; n=29)
Efficacy data based on long-term follow-up analysis (median time from ABECMA infusion to data cutoff 27.3 months [range: 24.1 to 33.1]; N=100). Data were generally consistent with the primary analysis.
Primary analysis data: sCR 28% (95% CI, 19-38), VGPR 25% (95% CI, 17-35), PR 19% (95% CI, 12-28).
Response is defined as achieving ≥PR. Of the 100 patients in the efficacy-evaluable population, 25 (25%) achieved a VGPR (95% CI, 16.5-33.5) and 18 (18%) achieved a PR (95%, 10.5-25.5).4
All complete responses were sCRs.
Based on a threshold of 10-5 using a ClonoSEQ® next-generation sequencing assay (NGS). MRD negativity was defined as the proportion of patients with ≥VGPR who are MRD negative at any time point within 3 months prior to achieving ≥VGPR until the time of progression or death.
CAR=chimeric antigen receptor; CI=confidence interval; mDOR=median duration of response; MRD=minimal residual disease; NE=not estimable; ORR=overall response rate; PR=partial response; sCR=stringent complete response; TTNT=time to next treatment; TTR=time to response; VGPR=very good partial response.